Moderna Vaccine Wikipedia
Sie enthält quasi einen Bauplan für ein Merkmal des Coronavirus. Modernas chief medical officer said the vaccine could be ready in January 2021.

Pfizer Biontech Covid 19 Vaccine Wikipedia
Spikevax tidigare COVID-19 Vaccine Moderna och mRNA-1273 är ett covid-19-vaccin som utvecklats 2020 av det amerikanska läkemedelsföretaget Moderna i samarbete med National Institute of HealthDet är det andra vaccin som fått godkännande av amerikanska Food and Drug Administration den 18 december dock endast för akutförsäljning.

Moderna vaccine wikipedia. Moderna is working to create mRNA medicines for a wide range of diseases and conditions. Januar 2020 meddelte Moderna om udviklingen af en vaccine til hæmning af COVID-19 coronavirus. Durch die Impfung gelangt dieser Bauplan über kleinste Fettpartikel.
Diese wird auch als Boten-RNA bezeichnet. Long-term storage temperature. These include potential new mRNA medicines for treating infectious diseases cancer rare diseases and cardiovascular disease.
The second dose of the PfizerBioNTech and Moderna vaccines can be administered up to six weeks after the first dose to alleviate a shortage of supplies. Ist ein in Cambridge im US-Bundesstaat Massachusetts ansässiges Biotechnologieunternehmen das sich auf die Entdeckung und Entwicklung von Arzneimitteln auf der Basis von Messenger-RNA konzentriert. Para a imunização é necessária a aplicação de duas doses.
Moderna grundades 2010 och utvecklar läkemedel baserade på budbärar. Mars 2020 hadde det amerikanske firmaet Moderna begynt med kliniske studier for deres vaksinekandidat mRNA-1273 i samarbed med Vaccine Research Center VRC ved National Institute of Allergy and Infectious Diseases NIAID som er del av National Institutes of Health NIH. Moderna has launched a trial of a vaccine to tackle the Beta variant or lineage B1351.
Ela é baseada na tecnologia mRNA RNA mensageiro e foi a segunda a ser aprovada nos Estados Unidos para uso emergencial. Another advantage of RNA vaccines is that since the antigens are produced inside the cell they stimulate cellular immunity as well as humoral immunity. Vaksinen mRNA-1273 inneholder budbringer-RNAet mRNA for det S-proteinet som koronaviraene.
Moderna Inc tidigare Moderna Theurapeutics är ett amerikanskt läkemedels- och bioteknikföretag baserat i Cambridge Massachusetts. Das Unternehmen stellt synthetische mRNA her die Patienten injiziert werden kann. If you experience a severe allergic reaction call 911 or go to the nearest hospital.
Impfstoffe wie Spikevax Vaccine Moderna von Moderna enthalten Genabschnitte des SARS-CoV-2-Virus in Form von messenger-RNA messenger-Ribonukleinsäure kurz mRNA. Modernas teknologi er en messenger RNA-forbindelse compound kaldet mRNA-1273 som hæmmer SARS-CoV-2 der koder for en form af virussens spike-proteinVaccinekandidaten er af den RNA-baserede vaccinetype og udvikles i et samarbejde mellem Moderna og US National Institute of. What should I do about side effects.
Call the vaccination provider or your healthcare provider if you have any side effects that bother you or do not go away. MRNA vaccines do not affect or reprogram DNA inside the cell. Sowohl der von BioNTech und Pfizer entwickelte Impfstoff Tozinameran als auch das von Moderna entwickelte Vakzin mRNA-1273 geben den Körperzellen eine mRNA -Vorlage zur Herstellung des Spike - Proteins von SARS-CoV-2 siehe RNA-Impfstoff.
The United States Food and Drug Administration gave Moderna permission to test the vaccine again in more people. Företagets enda kommersiella produkt är COVID-19 Vaccine Moderna. A Moderna Covid19-vakcinája kódnevén.
A mRNA-1273 - ou simplesmente Vacina Contra COVID-19 da Moderna - é uma vacina anti- covid-19 desenvolvida pela Moderna Therapeutics. The Moderna COVID-19 Vaccine is still being studied in clinical trials. The OxfordAstraZeneca COVID-19 vaccine sold under the brand names Vaxzevria and Covishield is a viral vector vaccine produced by the British University of Oxford British-Swedish company AstraZeneca and the Coalition for Epidemic Preparedness Innovations.
A Moderna COVID-19 vakcina engedélyezése Az mRNA-1273 közismertebb nevén Moderna COVID-19 vakcina egy SARS-CoV-2 koronavírus fertőzés elleni védőoltás amelyet a National Institute of Allergy and Infectious Diseases NIAID a Biomedical Advanced Research and Development Authority BARDA és a Moderna fejlesztett ki. The Moderna vaccine entered stage-three clinical trials on June 27 2020. On 17 February 2021 Pfizer announced neutralization activity was reduced by two-thirds for this variant while stating that no claims about the efficacy of the vaccine in preventing illness for this variant could yet be made.
MRNA-1273 egy amerikai harmadik generációs intramuszkuláris Covid19-vakcina melyet a Moderna biotechnológiai vállalat fejlesztett ki a National Institute of Allergy and Infectious Diseases NIAID a Biomedical Advanced Research and Development Authority BARDA és a Coalition for Epidemic Preparedness Innovations CEPI közreműködésével. Moderna utvecklar bland annat vaccin. Report vaccine side effects to FDACDC Vaccine Adverse Event Reporting.
The synthetic mRNA fragment is a copy of the specific part of the. Moderna designed their mRNA-1273 vaccine for COVID-19 in 2 days. The PfizerBioNTech COVID-19 vaccine can be kept between 25 and 15 C 13 and 5 F for up to two weeks before use and between 2 and 8 C 36 and 46 F for up to five days before use.
How The U S Government Bolstered Moderna S Covid 19 Vaccine Candidate Drug Discovery And Development

Covid 19 Vaccination In New Zealand Wikipedia
Moderna Has Admirers Still The Stock Gets Hit With Downgrades Barron S

Covid 19 Vaccination In Albania Wikipedia

Covid 19 Vaccination In The United States Wikipedia
Moderna Said Set To Send 1 Million Vaccines Next Week Cover Israel S Shortfall The Times Of Israel
Covid 19 Vaccination In Italy Wikipedia

Rna Vaccines A Novel Technology To Prevent And Treat Disease Science In The News
Covid 19 Vaccine Development Clinical Trials Gvn
Navigating The Search For A Covid 19 Vaccine In Houston My Vaccine Story Shows How Persistence And Luck Can Pay Off

6 Questions About Facial Swelling And Covid 19 Vaccines Answered Massdevice

Covaxin Covishield Mix Gives Positive Result In Icmr Study Global View What Experts Say

Coronavirus Update Aug 6 2021 Moderna Claims Its Vaccine Is 93 Percent Effective After Six Months Prague Czech Republic

Covid 19 Vaccine Development Clinical Trials Gvn
How Does Russia S Covid 19 Vaccine Compare With Pfizer Moderna Bloomberg

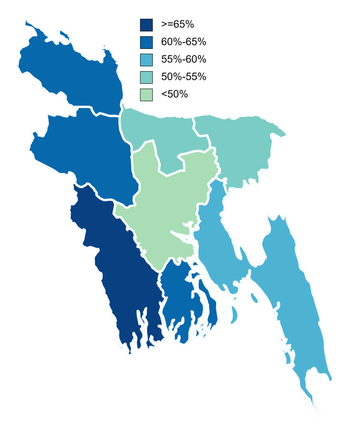


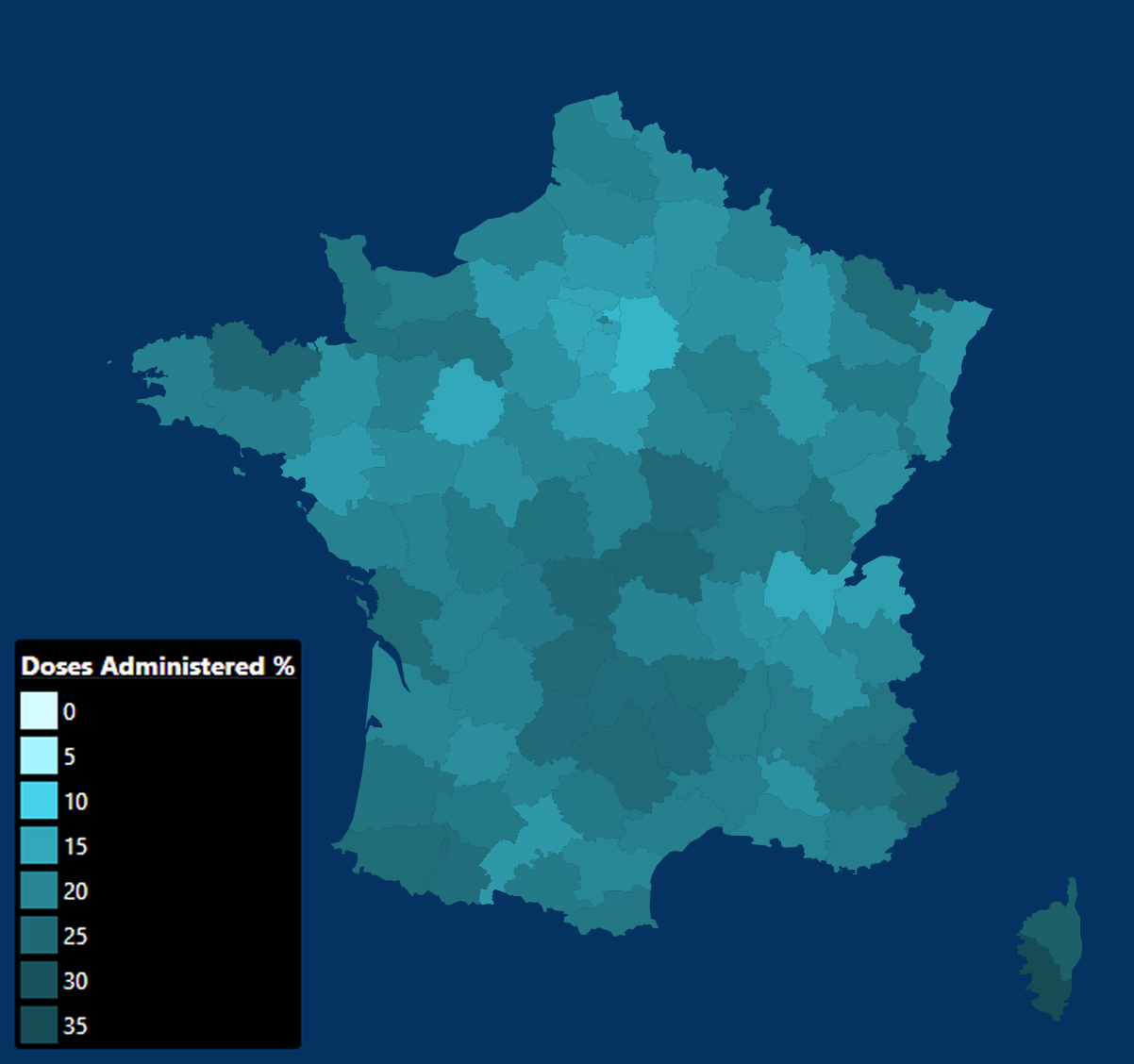
0 Response to "Moderna Vaccine Wikipedia"
Posting Komentar